What are PFO and ASD Closure Devices?
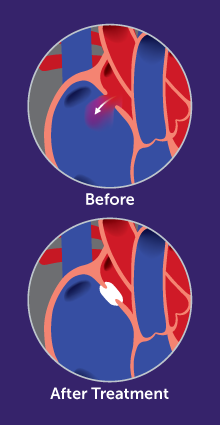 PFO/ASD closure devices are medical implants designed to close an Atrial Septal Defect (ASD) or Patent Foramen Ovale (PFO), two congenital heart conditions. These defects involve openings in the septum (wall) between the heart’s upper chambers. Closure devices are used in minimally invasive procedures to seal these openings, preventing abnormal blood flow between the atria.
PFO/ASD closure devices are medical implants designed to close an Atrial Septal Defect (ASD) or Patent Foramen Ovale (PFO), two congenital heart conditions. These defects involve openings in the septum (wall) between the heart’s upper chambers. Closure devices are used in minimally invasive procedures to seal these openings, preventing abnormal blood flow between the atria.
These devices come in various sizes and designs, often resembling small umbrella-like structures or disks. They are inserted through a catheter, a thin tube, which is guided to the heart. Once properly positioned, the closure device is deployed to cover and seal the ASD or PFO, promoting normal blood flow and reducing the risk of complications associated with these structural heart defects. The choice of device and procedure depends on factors, such as the defect’s size, location and individual patient characteristics.
What is Atrial Septal Defect (ASD)?
An Atrial Septal Defect (ASD) is a congenital heart condition characterized by a hole in the septum that separates the two upper chambers of the heart, known as the atria. This hole allows oxygen-rich blood from the left atrium to mix with oxygen-poor blood from the right atrium, potentially leading to an increased volume of blood being pumped to the lungs. ASDs can vary in size and severity, and while small defects might not cause significant symptoms or health issues, larger or untreated ASDs can strain the heart and lungs over time.
What is Patent Foramen Ovale (PFO)?
A Patent Foramen Ovale (PFO) is a common congenital heart anomaly characterized by a small hole in the septum that separates the atria. This hole, which is present since birth, typically closes shortly after birth but may remain open in some individuals. A PFO allows a small amount of blood to pass from the right atrium to the left atrium, bypassing the lungs. While PFOs often cause no symptoms and don’t require treatment, they have been associated with certain medical conditions, such as cryptogenic stroke (stroke with an unknown cause) and decompression sickness (the bends) in scuba divers, where blood clots or bubbles may pass through the PFO and reach the brain or other organs.
How do PFO/ASD Closure Devices work?
PFO/ASD closure devices are designed to address structural heart defects by effectively sealing openings in the septum between the heart’s upper chambers. The following outlines the basic steps of this minimally invasive procedure:
- It begins with the insertion of a catheter, a thin and flexible tube, into a blood vessel, often in the leg or groin. Once in, it is carefully guided through the blood vessels to reach the heart.
- Here, the closure device, typically made of biocompatible materials, is loaded onto the catheter. The catheter is then maneuvered so that the device is positioned precisely over the opening in the septum, either the ASD or PFO.
- The closure device is gradually released from the catheter. As it opens, the device’s specialized structure expands to cover the opening in the septum, effectively sealing it shut.
- Over time, the patient’s own tissue begins to grow over and around the closure device. This process, known as endothelialization, helps secure the device in place and promotes natural healing.
- With the closure device in position, blood flow between the heart’s upper chambers is redirected to its proper pathway, preventing abnormal circulation and reducing the risk of associated complications.
- As tissue continues to grow around the device, it becomes a permanent part of the heart’s anatomy, contributing to the normal functioning of the cardiac chambers.
What are the benefits of PFO/ASD Closure Devices?
PFO/ASD closure devices provide a minimally invasive alternative to open-heart surgery, resulting in shorter hospital stays, less postoperative discomfort, and quicker recovery times. Generally, one is able to enjoy a better quality of life and return to their normal activities sooner.
Additional benefits include:
- Reduced risk of cryptogenic strokes or decompression sickness in divers, as well as helps prevent recurrent medical events, such as repeated strokes
- Because the device becomes a permanent part of the heart’s anatomy, it offers ongoing protection, reducing the need for further interventions
- PFO/ASD closure devices come in various sizes and designs, allowing for a personalized approach that considers the specific characteristics of the defect and the patient’s unique anatomy. Plus they are suitable for a range of patients, from those with asymptomatic defects to those who have experienced related complications.
- Successful closure may eliminate the need for long-term medication regimens that were initially prescribed to manage associated risks.
- These devices have been extensively studied and have demonstrated their efficacy in preventing complications and improving patient outcomes.
While PFO/ASD closure devices offer significant benefits, their use is determined on a case-by-case basis. MemorialCare cardiac specialists will carefully evaluate each patient’s condition, medical history and risk factors to determine the most appropriate treatment approach for their specific needs.
What are the potential complications or side effects of PFO/ASD Closure Devices?
While PFO/ASD closure devices are generally safe and effective, like any medical procedure, they carry potential complications and side effects. These may include:
- Infection at the catheter insertion site or around the closure device
- Bleeding or bruising at the catheter insertion site
- Blood clots can form on the closure device, which may require blood-thinning medications
- In rare cases, the closure device may move from its intended position, requiring repositioning or removal
- Allergic reactions to materials used in the device
- The procedure might trigger irregular heart rhythms (arrhythmias)
- The catheter or closure device could potentially cause damage to blood vessels or heart tissue
- Despite the closure, a small amount of blood might still pass through the ASD or PFO due to incomplete sealing
- While rare, there’s a risk of clot formation during the procedure, which could lead to a stroke
- Reaction to anesthesia
- In a small number of cases, symptoms related to the ASD or PFO may persist or recur.
- Although uncommon, there could be long-term issues related to the implanted device, such as erosion, embolization or interference with adjacent structures
It’s important to note that the likelihood of experiencing these complications is relatively low, and the benefits of PFO/ASD closure devices often outweigh the risks, particularly for individuals with symptomatic defects or a history of related complications. Your MemorialCare cardiac specialists will carefully assess your individual risk and discuss with you any potential complications before proceeding with the procedure.
Regular follow-up appointments and adherence to post-procedure guidelines are important to monitor for any signs of complications and ensure the best possible outcomes.
Evaluation to be a candidate for PFO/ASD Closure Device is offered at:
To speak to the coordinator of the PFO/ASD Closure Device program, call:
FAQ about PFO/ASD Closure Devices
The main distinction between an Atrial Septal Defect (ASD) and a Patent Foramen Ovale (PFO) lies in the size and location of the openings in the heart’s wall between the atria (upper chambers) and its clinical impact. A PFO is a small opening that often closes after birth but may remain open, while an ASD is a larger and more significant hole in the atrial septum. PFOs are usually asymptomatic but can be linked to certain conditions like cryptogenic stroke (this refers to strokes for which no definite cause can be identified), while larger ASDs can strain the heart and lungs over time. Closure devices can be used to prevent abnormal blood flow and related complications.
Cardiac closure devices, used to treat conditions like Atrial Septal Defect (ASD) and Patent Foramen Ovale (PFO), are typically constructed from biocompatible materials designed to promote tissue integration and minimize adverse reactions within the body. These devices commonly incorporate materials such as nitinol, a shape-memory alloy that allows the device to be compressed for delivery through a catheter and then expands to its intended shape upon deployment. Nitinol’s unique properties ensure proper device positioning and stability. Additionally, closure devices may include a polyester or polytetrafluoroethylene (PTFE) fabric covering, enhancing tissue growth over the device and providing additional sealing capabilities. The biocompatibility of these materials contributes to the long-term success of the closure devices by minimizing the risk of adverse reactions or complications.
The symptoms of both Atrial Septal Defect (ASD) and Patent Foramen Ovale (PFO) can vary widely and may include fatigue, shortness of breath, palpitations and difficulty exercising. Some individuals with PFOs or small ASDs might remain asymptomatic and discover the condition incidentally during medical evaluations. However, larger ASDs can lead to more noticeable symptoms, such as recurrent respiratory infections, heart murmurs, or even signs of heart failure, like fluid retention and swelling. It’s essential for individuals experiencing any of these symptoms to seek medical evaluation, as proper diagnosis and management are crucial for maintaining heart health.
Cardiac closure procedures, used to treat conditions like Atrial Septal Defect (ASD) and Patent Foramen Ovale (PFO), are generally considered safe and effective. They are minimally invasive, catheter-based procedures that offer advantages such as shorter recovery times and reduced post-operative discomfort compared to open-heart surgery. While complications are rare, as with any medical intervention, there is a small risk of infection, bleeding or adverse reactions to anesthesia or the closure device. The decision to undergo a cardiac closure procedure is made based on a thorough evaluation of the individual medical history, symptoms and associated risks, in collaboration with a health care provider.
The recovery time after an ASD or PFO closure procedure is generally relatively short. Most patients can expect to stay in the hospital for a day or two after the procedure for observation. After discharge, individuals typically resume light activities within a few days and can gradually return to their normal routines over the following weeks. However, the exact recovery timeline can vary depending on individual factors and the specific procedure performed. Your health care providers will provide you with personalized guidance to ensure a smooth and safe recovery.
If left untreated, an Atrial Septal Defect (ASD) or Patent Foramen Ovale (PFO) can potentially lead to various health complications. Blood clots or bubbles can pass through the ASD or PFO, allowing them to travel to the brain and cause a stroke, particularly in the case of a PFO. Larger ASDs can strain the heart and lungs over time, potentially leading to heart failure, pulmonary hypertension, or other cardiovascular issues. Timely intervention, either through closure procedures or other appropriate management, is important to prevent these potential complications and maintain optimal heart health.